
Salivary Test for Early and Reliable Diagnosis of Endometriosis

What is Ziwig Endotest® ?
Ziwig Endotest® is an in vitro diagnostic medical device, CE IVD marked, from the company Ziwig, available by medical prescription, for the early diagnosis of endometriosis based on a saliva sample.
This innovative and non-invasive diagnostic tool is grounded in the analysis of salivary micro-RNAs through High-Throughput Sequencing (NGS) and Artificial Intelligence (AI).
It has been validated through one of the largest clinical trials conducted in this field, in collaboration with 18 specialized French centers in endometriosis [1].
When to prescribe the salivary test ?
In the presence of suggestive symptoms of endometriosis [2], taking the form of chronic pelvic pain, possibly accompanied by:
- Dysmenorrhea
- Deep dyspareunia
- Painful urination/micturition
- Painful defecation/dyschezia
- Painful rectal bleeding or hematuria during menstruation
- Shoulder tip pain during menstruation
- Infertility

What are the advantages of Ziwig Endotest® ?
Limitations of the test
- The patient must be at least 18 years old and a maximum of 43 years old at the time of the test.
- The patient should have no history of cancer or infection with the human immunodeficiency virus (HIV).
- The patient should not be pregnant at the time of the test.
Interpretation of results
- Positive result : Indicates the presence of endometriosis in the patient.
- Negative result: Indicates the absence of endometriosis in the patient.
- Invalid result : The sample and/or sequencing could not be analyzed or is not interpretable
Where to perform the sample collection for Ziwig Endotest® ?
BIONEXT offers 3 options for residents or cross-border patients.
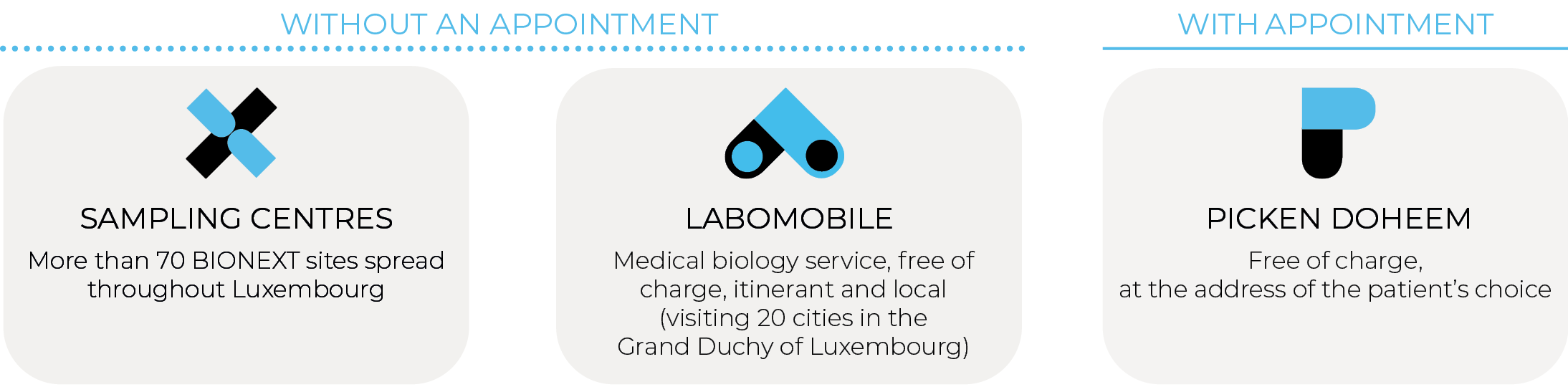
The test is currently not covered by the CNS (National Health Fund), please consult the laboratory for the out-of-nomenclature pricing.
Consult our catalog of analyses
Treatment and care
Today, there is no treatment capable of completely curing endometriosis. However, early diagnosis allows for targeted management that may slow down or even halt the worsening of pain and other symptoms. Additionally, infertility treatment can be optimized, and the quality of life for patients improved.
Beyond the tailored pain management for each patient and the necessary supportive care, hormonal treatments, relying on the use of combined oral contraceptives or progestins, aim to suppress menstruation. The standard treatment limits or reduces the intensity of symptoms and their impact on the quality of life.
As a second-line option, it is also possible to turn to Gn-RH analogs, inducing a reversible state of menopause in the patient. This treatment is always accompanied by 'add-back therapy' to prevent hot flashes.
Surgical treatment is considered when medical interventions prove insufficient to alleviate pain [8]. It aims to remove or electrocoagulate endometriotic lesions, with the procedure often performed through laparoscopy
Sciences
Ziwig Endotest® utilizes two cutting-edge technologies: high-throughput sequencing and artificial intelligence. With a simple saliva sample, 109 different micro-RNAs are analyzed to diagnose endometriosis.
The development of a non-invasive test for the diagnosis of endometriosis has been the subject of intense medical research for many years. Over 100 biomarkers have been evaluated during the past decades.
Among them, a new class of molecules described for the first time in 1993, micro-RNAs, has emerged as a promising option, supported by an increasing number of studies on tumors and neurodegenerative disorders. Micro-RNAs are small non-coding RNAs. They play a role in gene expression: when a micro-RNA binds to its target, a specific messenger RNA, it either inhibits or stimulates its translation into a protein and/or induces its destruction. Micro-RNAs are also released into the extracellular environment through various transport structures that protect them from ribonucleases in the circulation, giving them remarkable stability. These circulating micro-RNAs are found in variable quantities in bodily fluids (blood, urine, breast milk, tears, saliva, etc.).
In recent years, evidence of the contribution of microRNAs to the pathophysiological mechanisms of endometriosis has been accumulating. A direct link has been proven between the dysregulation of certain micro-RNAs and the development of endometriotic lesions [9].
Get in touch with our medical department.
The research on Ziwig Endotest® has been published in peer-reviewed international scientific journals.
Bibliography
[1] Endometriosis Associated-miRNome Analysis of Blood Samples:A Prospective Study
Bendifallah S., Dabi Y., Suisse S., Delbos L., Poilblanc M., Descamps P., Golfier F., Jornea L., Bouteiller D., Touboul C., Puchar A. et Daraï E. I Mai 2022
The aim of our study was to describe the bioinformatics approach to analyze miRNome with Next Generation Sequencing (NGS) of 200 plasma samples from patients with and without endometriosis. Patients were prospectively included in the ENDO-miRNA study that selected patients with pelvic pain suggestive of endometriosis.
https://ziwig.com/wp-content/uploads/2022/05/diagnostics-12-01150.pdf
[2] ESHRE Guideline Endometriosis
Guide 2022
https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Endometriosis-guideline
[3] Machine learning algorithms as new screening approach for patients with endometriosis
Bendifallah S., Suisse S., Puchar A., et al. I Janvier 2022
Endometriosis—a systemic and chronic condition occurring in women of childbearing age—is a highly enigmatic disease with unresolved questions. While multiple biomarkers, genomic analysis, questionnaires, and imaging techniques have been advocated as screening and triage tests for endometriosis to replace diagnostic laparoscopy, none have been implemented routinely in clinical practice.
https://www.nature.com/articles/s41598-021-04637-2
[4] Clues for Improving the Pathophysiology Knowledge for Endometriosis Using Serum Micro- RNA Expression.
Dabi Y & al. Diagnostics (Basel). 2022 Jan 12;12(1):175. doi:10.3390/diagnostics12010175.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8774370/pdf/diagnostics-12-00175.pdf
[5] Salivary MicroRNA Signature for Diagnosis of Endometriosis
Bendifallah S., Suisse S., Puchar A., et al. I Janvier 2022
Background: Endometriosis diagnosis constitutes a considerable economic burden for the healthcare system with diagnostic tools often inconclusive with insufficient accuracy. We sought to analyze the human miRNAome to define a saliva-based diagnostic miRNA signature for endometriosis
[6] MicroRNome analysis generates a blood-based signature for endometriosis.
Bendifallah S & al. Sci Rep. 2022 Mar 8;12(1):4051. doi: 10.1038/s41598-022-07771-7
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8902281/pdf/41598_2022_ Article_7771.pdf
[7] Validation of the Salivary miRNA Signature of Endometriosis — Interim Data
Bendifallah S., Dabi Y., Suisse S., et al. | Juin 2023
The discovery of a saliva-based micro–ribonucleic acid (miRNA) signature for endometriosis in 2022 opened up new perspectives for early and noninvasive diagnosis of the disease. The 109-miRNA saliva signature is the product of miRNA bio- markers and AI modeling. We designed a multicenter study to provide external validation of its diagnostic accuracy. We present here an interim analysis.
https://evidence.nejm.org/doi/full/10.1056/EVIDoa2200282
[8] Haute Autorité de Santé. Prise en charge de l’endométriose. Fiche de synthèse. Décembre 2017
[9] Panir K et al. Hum Reprod Update 2018;24(4):497-515
Bibliographie complémentaire :
Saliva microRNA signature to diagnose endometriosis: A cost-effectiveness evaluation of the Endotest®
Ferrier C., Bendifallah S., Suisse S., Dabi Y., Touboul C., Puchar A., Zarca K. et Durand Zaleski I Novembre 2022
Objective : To evaluate a saliva diagnostic test (Endotest®) for endometriosis compared with the conventional algorithm. Design: A cost-effectiveness analysis with a decision-tree model based on literature data. Setting: France. Population: Women with chronic pelvic pain.
https://pubmed.ncbi.nlm.nih.gov/36424910/
Non-coding RNAs in endometriosis: a narrative review
Panir K et al. | July 2018
https://doi.org/10.1093/humupd/dmy014
Endometriosis-associated infertility diagnosis based on saliva microRNA signatures
Dabi Y., Suisse S., Puchar A., Delbos L., Poilblanc M., Descamps P., Haury J., Golfier F., Jornea L., Bouteiller D., Touboul C., Daraï E. et Bendifallah S. I Octobre 2022
Research question: Can a saliva-based miRNA signature for endometriosis-associated infertility be designed and validated by analysing the human miRNome? Design: The prospective ENDOmiARN study (NCT04728152) included 200 saliva samples obtained between January 2021 and June 2021 from women with pelvic pain suggestive of endometriosis.
https://www.rbmojournal.com/article/S1472-6483(22)00714-3/fulltext

